Inspired by Nature,
We’re reimagining
antibiotics
Developing a new class of ribosome-targeting antibiotics
About Us
Kinvard Bio, a spin-out from the prestigious Myers Lab at Harvard University’s Department of Chemistry and Chemical Biology, is pioneering the development of a new and differentiated class of ribosome-targeting antibacterials known as oxepanoprolinamides (OPPs).
With a focus on addressing the highest unmet patient need and commercial potential, Kinvard Bio’s OPP class of antibacterials presents substantial therapeutic potential across multiple clinical indications.
Designed for both intravenous and oral routes of administration, our OPPs offer versatile treatment options for a range of acute and chronic bacterial infections.
Key technology features:
- Proprietary synthetic chemistry platform: Robust, scalable chemistry
- Clinically validated target: The OPPs are structurally preorganized for optimal binding in the bacterial ribosome
- Broad spectrum activity: Effective against a broad range of clinically important pathogens, including Gram-postive and Gram-negative bacteria, including non-fermenting Gram-negative bacilli (NFGNB)
- Resistance avoidance: OPPs overcome a wide range of pre-existing resistance mechanisms
- Targeting acute and chronic infections: Combined IV / oral routes of administration and extended spectrum of activity affords significant clinical indication optionality
oxepanoprolinamides (OPPs)

A GLOBAL NEED
Bacterial Infections: A Major Contributor to the Global Burden of Disease
The rising incidence of antimicrobial resistance (AMR) is diminishing the effectiveness of existing oral antibiotics. With few new oral treatments in development, patients are increasingly dependent on hospital-based intravenous therapies, driving up healthcare costs and worsening patient outcomes.
deaths
are linked to bacterial infection.1
Deaths each year since 1990 due to antibiotic resistance.
The rising incidence of drug-resistant infections is projected to claim over 39 million lives by 2050.2
Chronic bacterial infections are a leading cause of mortality and morbidity.
Mortality rates for the chronic respiratory infection, NTM-lung disease are between 10-48% in the US; averaging 27% globally with recurrence rates as high as 41%.
Infections are the second leading cause of death in patients with cancer and are often caused by resistant bacteria.
Resistance rates are up to 5x higher for key pathogens from cancer outpatients as compared to non-cancer patients.3
OUR PLATFORM
Creating a new class of ribosome-targeting antibiotics
The oxepanoprolinamide (OPP) platform
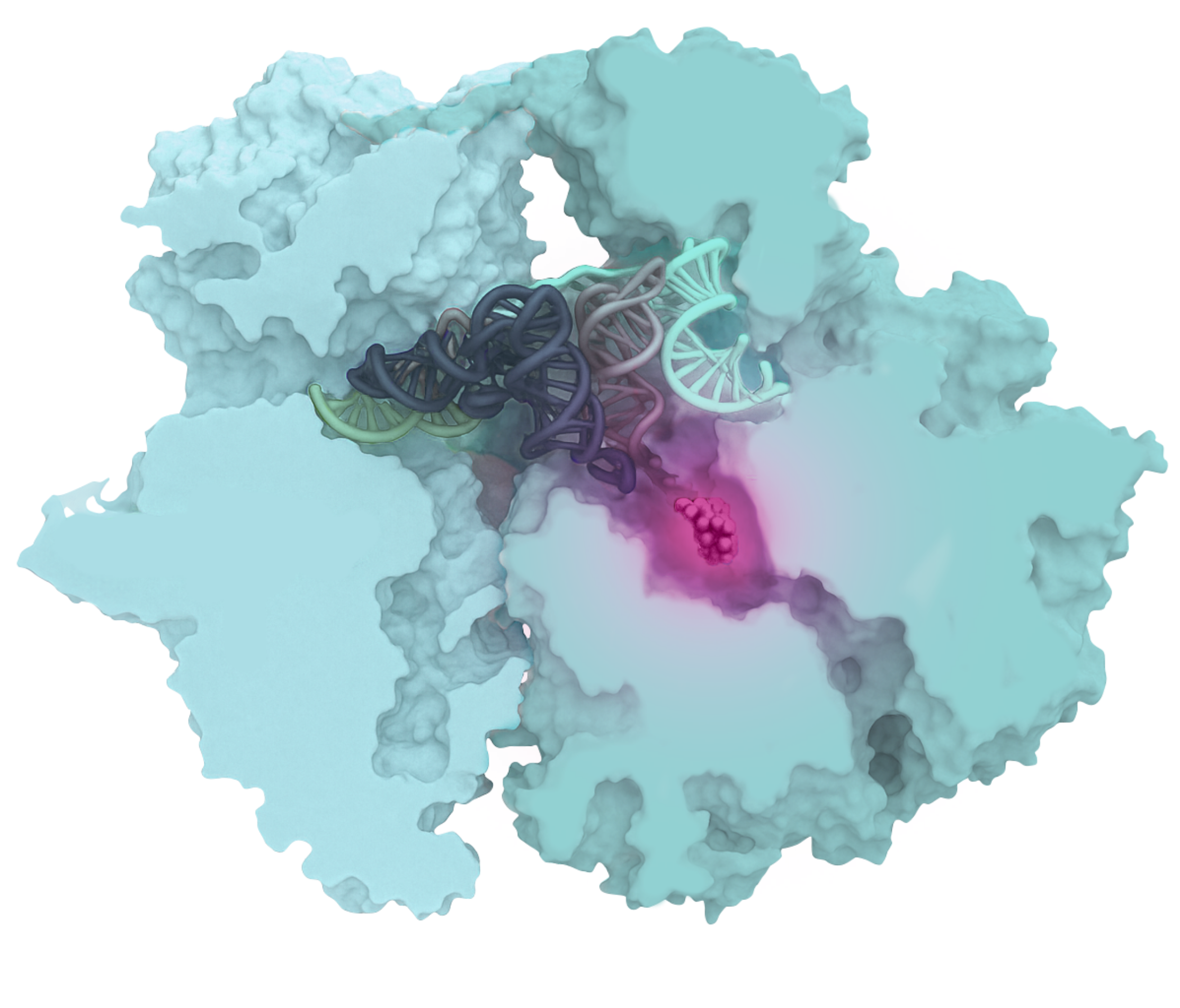
Oxepanoprolinamides (OPPs) feature a unique molecular structure that ensures optimal binding within a specific site of the bacterial ribosome. This results in potent, broad-spectrum antibacterial activity, both in vitro and in vivo, while effectively bypassing a wide range of pre-existing resistance mechanisms.
OPPs bind optimally within the Peptidyl Transferase Center (PTC) of the ribosome, disrupting protein synthesis and rendering bacteria non-viable.
The novel bridged macrobicyclic ring system of OPPs ensures optimal binding within the ribosome’s Peptidyl Transferase Center (PTC), resulting in potent antibacterial activity and excellent drug-like properties.
OPPs exhibit up to 300-fold greater binding affinity and potency compared to traditional ribosome-targeting antibiotics like clindamycin. This enhanced target engagement results in potent antibacterial activity against a broad range of clinically important bacterial pathogens.
The OPPs exhibit excellent in vitro and in vivo activity against both Gram-positive and Gram-negative pathogens, including challenging multidrug-resistant and pan-drug-resistant bacteria. The compound class also demonstrates activity against nontuberculosis mycobacteria (NTM) associated with difficult to treat chronic respiratory infections.
The structural framework of OPPs is preorganized for optimal binding within the bacterial ribosome. This design effectively ‘bypasses’ a broad range of diverse, pre-existing resistance mechanisms, offering promising potential for clinical impact in drug resistant infections.
The OPPs offer excellent potential for both oral and intravenous drug delivery, addressing the critical need for IV-oral step-down therapies. Dual routes of administration will reduce hospitalizations, shorten inpatient care durations, and effectively manage chronic bacterial infections.
The OPP chemistry platform offers significant expansion and synthetic scalability, allowing us to build our pipeline into new programs and clinical indications.
OUR PIPELINE
A Pipeline Focused on Patient Need and Commercial Potential
Tackling challenging acute and chronic bacterial infections
Kinvard Bio leverages advanced rational drug design to create a novel class of therapeutics that target the bacterial ribosome. By engaging a clinically validated target in a new way, Kinvard Bio aims to tackle the global challenges of both acute and chronic bacterial infections, as well as antimicrobial resistance.
Lead Optimization (LO) stage: Hospital-Acquired and Ventilator-Acquired Bacterial Pneumonia (HABP/VABP) and complicated Urinary Tract Infections (cUTI). This focus on Gram-negative bacteria addresses some of the most challenging and resistant pathogens in healthcare.
Advancing through lead optimization (LO) towards the Investigational New Drug (IND) stage for Community-Acquired Bacterial Pneumonia (CABP) and Acute Bacterial Skin and Skin Structure Infections (ABSSSIs). Our efforts target all major CABP/ABSSSI pathogens, including those resistant to known antibiotics.
Our discovery stage program is progressing the OPPs in additional clinical indications associated with high unmet patient need and commercial potential. This includes chronic respiratory diseases such as nontuberculosis mycobacterial lung disease (NTM-LD).